Published online Nov 10, 2015. doi: 10.5317/wjog.v4.i4.86
Peer-review started: July 27, 2015
First decision: September 17, 2015
Revised: September 27, 2015
Accepted: October 16, 2015
Article in press: October 19, 2015
Published online: November 10, 2015
Increasing multi-ethnicity in countries endemic or non-endemic for hemoglobinopathies has brought fundamental changes to the screening strategies for these traits. While in the past pre-screening on microcytosis was a reasonable method to economize upon follow up analysis, selecting low mean corpuscular volume means today missing all those normocytic carriers of common traits associated with severe conditions. Therefore, blood count should not be considered as a pre-selection tool but as additional information to be used for the interpretation of the provisional results, obtained by routine high throughput separation and measurement of the hemoglobin (Hb) fractions. Moreover, the moment of screening should be well planned depending on the social and cultural situation. Screening for genetic diseases in a modern multi-ethnic society should be offered to couples seeking progeny when both partners are more likely to be equally concerned with the good health of their children. In several societies screening before marriage and changing partner choice is culturally accepted. However, new generations are bound to disagree with these more or less imposed conditions and may decide not to renounce the choice of their partner asking for other preventive methods. In addition, a carrier state during pre-marital screening may in some cultures stigmatize the carrier, mostly the female with adverse social consequences. Therefore, screening for hemoglobinopathies early in pregnancy is the most sensible alternative in modern countries. Adding hemoglobinopathies to the routine rhesus screening using a simple separation of the Hb fractions on dedicated devices (high performance liquid chromatography or capillary electrophoresis) will virtually identify all female carriers of all common traits responsible for the severe conditions mainly sickle cell disease and thalassemia major in time for partner analysis, counseling and primary prevention.
Core tip: All women in most modern countries are offered screening for rhesus antagonism and infectious diseases early in pregnancy. Hemoglobinopathy (HBP) screening done together with rhesus screening using an inexpensive routine high performance liquid chromatography or capillary electrophoresis analysis could identify all women carriers of the frequent traits associated with the severe forms of HBP. Subsequent partner analysis could identify all couples at risk to be confirmed by molecular analysis in time for prenatal diagnosis when requested. This procedure will allow the prevention task to be offered at the most logical moment and by the most specialized hands of the gynecologists and obstetricians in collaboration with the local labs and the genetic counselors.
- Citation: Giordano PC. Universal screening for hemoglobinopathies in today's multi-ethnic societies: How and when. World J Obstet Gynecol 2015; 4(4): 86-94
- URL: https://www.wjgnet.com/2218-6220/full/v4/i4/86.htm
- DOI: https://dx.doi.org/10.5317/wjog.v4.i4.86
While up to a recent past were the Mediterranean countries those concerned with endemic hemoglobinopathy (HBP), with the thalassemia syndromes being the almost exclusive traits to be identified, today both Southern and Northern Europe are becoming a melting pot of different cultures from Africa, Middle and Far East, with different HBP traits to be identified[1]. In fact the same ethnic changes are observed all over the world and without prevention strategies many immigration countries will be confronted with a dramatic increases of severe HBP leading to human suffering and to increased costs of public health for the management of these incurable and expensive diseases[2,3]. To prevent all this, a broader spectrum screening for relevant HBP would have to be done in the endemic countries of Southern Europe and new national screening and managing strategies would have to be introduced in all other non-endemic countries experiencing massive immigration[4].
Over the past decades, regular beta thalassemia trait screening was based upon pre-selection of healthy but slightly anemic and microcytic carriers by measuring their low mean corpuscular volume (MCV) eventually persisting after iron therapy. This pre-selective screening method was considered practical and economically justifiable due to the fact that a complete blood count (CBC) was less expensive than screening by hemoglobin (Hb) electrophoresis and manual measurement of the HbA2 fraction[5]. This strategy might still be practical for large population screening campaigns for thalassemia in emerging countries. However, for screening and regular diagnostics in (non-endemic) developed countries this pre-selective strategy is inefficient and not cheaper at all.
In fact, even if well applied, this procedure implies at least two visits of the anemic carrier to the general practitioner (GP), two CBC’s and at least one iron prescription. Moreover, healthy but slightly anemic thalassemia carriers are often kept on iron therapy for a long time before the hemoglobinopathy option is considered and investigated. In this scenario, delayed diagnosis of beta thalassemia carrier status in couples at risk might easily happen, resulting in the birth of a first severely affected child.
To avoid this unfortunate way of (mis) managing diagnostics and prevention, carrier screening campaigns at school, before obtaining the marriage certificate and before or early in pregnancy have been organized for several decades in most endemic Mediterranean countries, but often following the same strategy, being CBC as the first step followed by Hb separation and HbA2 measurement in case of low MCV[6-13].
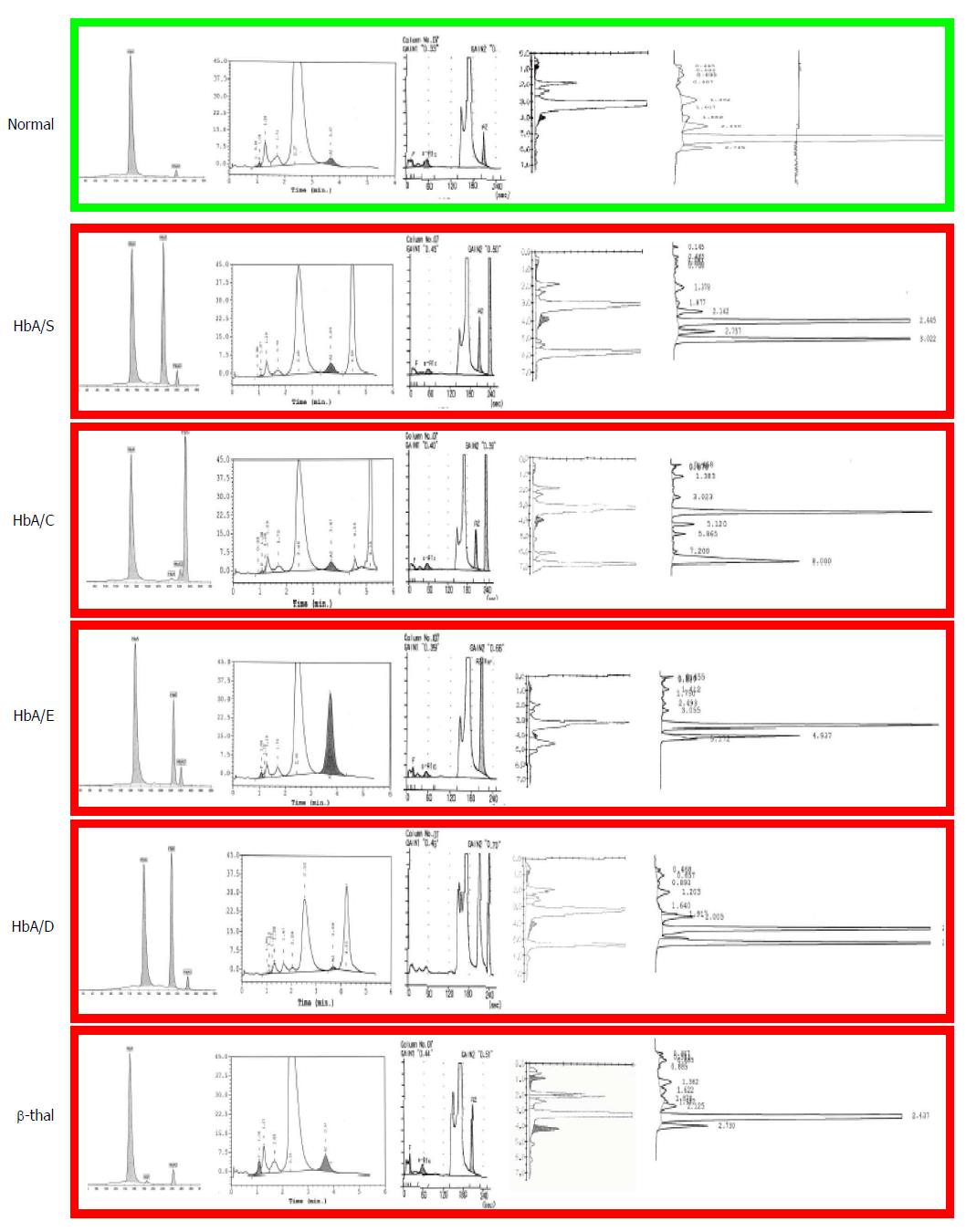
Today these methods are inappropriate. Dedicated high performance liquid chromatography (HPLC) and/or capillary electrophoresis (CE) devices are available in all modern laboratories where separation and estimation of all normal and abnormal Hb fractions is obtained automatically (Figure 1). These automatic methods are much faster and cheaper to public health than the procedure mentioned above, involving GP visits, inadequate iron therapies and repetitions of non-conclusive laboratory analyses. Moreover, carriers of common traits like HbS and HbC or less common traits like HbDPunjab or HbOArab, all associated with sickle cell disease (SCD), are not anemic or microcytic unless an independently inherited alpha thalassemia trait is also present. As a matter of fact, those few SCD carriers diagnosed today are identified just by chance, because of their border line MCV due to a coexisting alpha + thalassemia trait (-α/αα) or because of diabetes control with HbA1c later in life. Moreover, carriers of HbE, at risk for both severe beta thalassemia and SCD in their progeny, are often just border line microcytic, especially if vitamin B12 deficiency is also present. Therefore, pre-selecting by MCV in a multi-ethnic society, means missing the majority of the carriers of relevant traits associated with severe conditions in the progeny (Table 1). Screening should also not be based upon ethnic origin selection but should be offered at the national level and at the most logical moment. Ethnic selection has been matter of discussion since HBP in some non-endemic European countries has been considered as a rare “import” disease, too infrequent in the general population to justify universal screening. Indeed HBP are not found very frequently in the so called “low risk” North-European populations but low risk is not “no risk” and carriers of beta thalassemia, alpha thalassemia and HbDPunjab are regularly diagnosed in North-Europeans and not only because of distant (forgotten) “ethnic ancestors” but also due to “local” mutations. In fact, selecting for ethnic origin means discriminating both the included and the excluded ones, and adds to the risk when having found a carrier in the high risk population, the low risk partners in mixed couples are not checked, just supposing that for them the chance of being a carrier is “very low”.
| β traits | β minor | HbS | HbE | HbC | HbD | HbY |
| β minor | β major | |||||
| HbS | SCD | SCD | ||||
| HbE | β major | SCD | β minor | |||
| HbC | β minor? | SCD | β minor | β minor | ||
| HbD | β minor | SCD | β minor | Normal | Normal | |
| HbX | ? | ? | ? | ? | ? | ? |
Because of all these considerations newborn screening (NBS) is universal in most countries that offer this option. However, NBS is not providing primary prevention and not even retrospective primary prevention to couples at risk if they are not properly counseled[4,5,14].
For primary prevention, in the new multi-ethnic societies, screening should be offered to couples at risk before the first affected child is born and how and when universal screening should be organized starting with separation and measurement of the Hb fractions on HPLC or CE and using CBC as auxiliary data is further explained in this review.
To understand the HPLC/CE and CBC results one should keep in mind that thalassemias and abnormal hemoglobins are both HBP.
Thalassemias are caused by mutations on the globin genes that impair the expression of the genes reducing the amount of globin which is needed to form normal hemoglobin. This explains the microcytic hypochromic state of the red cells, the typical erythromorphology and the qualitatively normal HPLC/CE separations.
Abnormal hemoglobins (like the common HbS, HbC, HbE and HbD) are caused by mutations on the (beta) globin genes that change the structure of the globins and herewith of the more or less normally expressed abnormal hemoglobins. This explains the normocytic normochromic state of the red cells and the abnormal HPLC/CE separation. An exception among the common variants is HbE which is an abnormal hemoglobin with lower expression (approximately 25%) with a mild thalassemic phenotype in the carrier.
The genes involved in Hb expression during pre and postnatal life are the embryonic epsilon and zeta (ε and ζ), the fetal gamma (γ), the full expression beta (β) and alpha genes (α) and the low expression delta (δ) genes. All Hb molecules expressed during embryonic, fetal and postnatal life are tetramers. From the fetal life on, these tetramers are formed by 2 alpha and 2 non-alpha like globin chains. In normal conditions, the expression of postnatal hemoglobin HbA (α2/β2) and HbA2 (α2/δ2) is coded by two β-globin genes (one of paternal and one of maternal origin) located on chromosome 11, four α-genes (two of maternal and two of paternal origin) located on chromosome 16 and two δ genes, adjacent to the β-genes. The four γ genes needed for fetal HbF (α2/γ2) expression during fetal life, are silenced during the first year of life. Therefore the expected expression of the Hb fractions in normal adults will be approximately 96%-97% HbA 2.5%-3% HbA2 and 0.5%-1% HbF. All changes found in these parameters can be associated with a relevant hemoglobinopathy.
Materials and methods for “basic” HBP carrier screening are very simple and available in virtually all laboratories[15]. Screening should always include separation and estimation of the Hb fractions on automated HPLC or CE and the separation results should be compared for compatibility with the CBC parameters. Samples should be collected in EDTA and be processed directly for the best CBC and Hb separation results. However, samples kept refrigerated and processed a few days later are still valid for separation and measurement of the fractions and for DNA extraction if required. Dry samples, as eventually used for newborn screening, are suitable for obtaining a provisional result from the separation on HPLC or CE but are obviously not suitable for CBC and DNA extraction from dry samples requires special methods.
Separation and measurement of the Hb fraction has been done automatically for more than two decades using dedicated devices. The most popular HPLC and capillary electrophoresis devices (CE) evaluated by Van Delft et al[16] are available in virtually all laboratories.
These devices identify rapidly and at very low cost all carriers of high HbA2 beta thalassemia and of the common and relevant HbS, HbC, HbE and HbD which will represent most of the positive results to be expected in a multi-ethnic population[16].
HbA2 levels above 4% are diagnostic for beta thalassemia trait with HbF levels that may or may not be slightly elevated[15]. Some cases of beta thalassemia trait may however present with HbA2 levels lower than 4%. In these cases, if the CBC remains microcytic, alpha and beta thalassemia should be further investigated at the molecular level, especially when risk is suspected in a putative carrier couple. In some cases, interfering delta globin gene defects may cause low HbA2 (δ2/α2) levels and especially delta thalassemia may interfere with the diagnosis of high HbA2 beta thalassemia[17].
Carriers of the common Hb variants (HbS, HbC, HbE and HbD) are identified at 100% sensitivity and over 95% specificity (Figure 1)[16]. The high specificity is partially due to the precise positions in which these fractions migrate but mainly due to the fact that these variants are the most common. To exclude rare variants moving in the same position as HbS the fraction can be confirmed with 100% specificity using the sickle test[18]. For all presumed couples at risk confirmation should be performed at the molecular level. All rare variants not corresponding to the common one should also be investigated at the molecular level to exclude genetic risk.
Screening with HPLC/CE methods will practically identify all carriers of “beta gene defects” that are important for offering primary prevention of severe hemoglobinopathies in a multi-ethnic society. Adult carriers of mild alpha gene defects are not identified on HPLC/CE but can be easily identified during newborn screening by the presence of Hb Bart’s (Table 2).
| Genotype | α-thalassemia | Phenotype | MCV | HPLC | HbH (adult) | Genetic risk for the |
| Condition name | MCH | CE | Hb Bart’s (newborn) | progeny of carriers | ||
| αα/αα | Normal | Normal | Normal | Normal | Absent | None |
| Absent | ||||||
| -α/αα | α+ heterozygous | Eventually anemic | borderline | Normal | Absent | HbH |
| 0%-5% | ||||||
| -α/-α | α+ homozygous | Mildly anemic | Low | Normal | Absent | HbH |
| HbA2↓ | 5%-10% | |||||
| --/αα1 | α° heterozygous | Mildly anemic | Low | Normal | Absent | HbH or Hb Bart’s HF |
| HbA2↓ | 5%-10% | |||||
| --/-α2 | HbH disease | Intermediate | Lower | HbH | 0%-10% | HbH or Hb Bart’s HF |
| hemolytic anemia | Hb Bart’s | 10%-30% | ||||
| HbA2↓↓ | ||||||
| --/-- | Hb Bart’s | Severe intrauterine | Severe | No HbA or HbF in newborn | Hb Portland | Lethal to newborn. |
| Hydrops Fetalis | hemolytic anemia | morphology | Hb Bart’s | Life threatening for mother |
Due to the lethal outcome of homozygous alpha zero defects and the usually mild to intermediate phenotype of HbH disease, carriers of alpha gene defects represent a relatively less severe problem to public health. However, and also because of maternal risk, screening for these traits, common in Southeast Asian and Chinese populations, should be taken into serious consideration (read further).
The CBC parameters should be compatible with the HPLC or CE results. As mentioned above, carriers of thalassemia are characterized by a mild microcytic (MCV↓) hypochromic (MCH↓) anemia (Hb↓) not improving on iron therapy. Conversely, carriers of the common Hb variants, separable on HPLC or CE are not microcytic, unless also carriers of coexisting alpha thalassemia. In addition, a low MCV value tends to increase with time and in case of borderline values measured often in mild alpha thalassemia, one can better rely on the MCH value which remains more constant. The best discriminating CBC parameter to differentiate between iron deficiency and thalassemia will be the red blood cell (RBC) count, which is often elevated in the compensated beta thalassemia carrier with sufficient folic acid intake. Indicative parameters are summarized in Table 3.
The microcytic anemic condition is less evident in carriers of mild alpha thalassemia. This depends from the presence of 4 alpha genes and from the number of alpha genes left active. CBC can then be nearly normal in the mildest alpha thalassemia forms and strongly abnormal in the severe HbH disease form (Table 2).
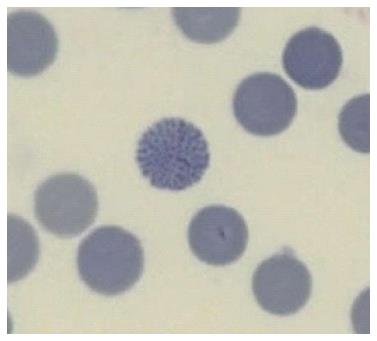
While often disregarded in modern laboratories because time consuming and because it requires trained observers, the smear gives away the diagnosis of thalassemia to the expert eye who will identify the typical erythromorphology of the target cells and anisopoikylocytosis (last column in Table 3), the last being the visual equivalent of the differential parameter “red cell distribution width” (RDW). Moreover, smears stained with supravital brilliant cresyl blue (reticulocytes staining), will identify the rare HbH inclusion body cells, specific to the alpha zero thalassemia carrier (at risk for hydrops fetalis in the progeny) (Figure 2).
As mentioned above, an adult β-thalassemia carrier will usually show 4% or more HbA2. Then, if matching with the expected microcytic hypochromic CBC parameters, the provisional diagnosis will be “carrier of β-thalassemia”. However, some rare β-thalassemia mutations show HbA2 values in the “grey zone” (3.5%-3.9%) or even normal levels. In these cases excluding a β-thalassemia trait because of a value just below the normal cut of, trusting on the presumed precision of the measurement is very imprudent, especially when CBC indicates persistent microcytosis in absence of iron depletion. In these cases further investigation is needed as one may have a borderline HbA2 β-thalassemia, an α-thalassemia or a large β deletion thalassemia defect[19]. Some HbF (up to approximately 7%-8%) will eventually be present in the beta thalassemia carrier. Higher HbF levels are usually associated with delta/beta thalassemia deletion defects that will present with normal HbA2 levels and masked microcytosis due to the larger size of the HbF cells.
Dedicated devices may overestimate or underestimate the HbA2 levels in the presence of Hb variants. However, measurement of HbA2 in the presence of HbA and any β variant is obsolete. When both HbA and the β variants are expressed (in non-transfused patients) it means that both β genes are expressed and that there is no need to measure HbA2 to establish the presence of a non-expressed β thalassemia gene.
Adult α-thal carriers will show no specific characteristics on HPLC or CE except for a marginal reduction in HbA2 expression. The only exception is HbH disease, which will usually present with significantly low HbA2 and a rapid migrating or eluting but unstable and quickly disappearing HbH fraction.
When suspecting α-thal because of the CBC parameters in the absence of iron depletion and low HbA2, then molecular analysis is needed to detect the common deletions or point mutations. On the other hand, α-thal can easily be detected at birth by the presence of Hb Bart’s. The presence of Hb Bart's observed during newborn screening should be reported to avoid a more laborious diagnosis later in life.
In the presence of a single non active α gene Hb Bart’s value between 0% to 5% is usually measured. The rates rises from 5% to 10% when two α genes are compromised (either in cis --/αα or trans -α/-α), while in HbH disease (-α/--), Hb Bart’s level can go from 10% to 30%. In case of the lethal hydrops fetalis syndrome (--/--) no HbF is present in the fetus or in the severely compromised newborn but traces of HbH (β4), Hb Bart’s (γ4), (ε4), Hb Gower-I (ζ2ε2) and the embryonic Hb Portland (ζ2γ2) are detected (Table 2).
Together with iron are vitamin B12 and folic acid essential for erythropoiesis. Folic acid is prescribed before and during pregnancy also to prevent neural tube defects like spina bifida. Folic acid is a vitamin with limited body storage that can be rapidly exhausted during increased cell division, a condition not only present in pregnancy but also chronic in thalassemia carriers. As mentioned above, the RBC count tends to be elevated in well compensated beta thalassemia carriers. Compensation is folic acid dependent and anemia may become more pronounced in beta thalassemia carriers in case of low folic acid intake. If folic acid is sufficiently present, RBC counts tend to be higher and by this compensatory mechanism Hb levels tend to be higher as well, while the packed red cells (PCV) value remains within normal levels. Measurement of serum folic acid gives no clue since it is the limited reserve of this vitamin which determines maintenance of the compensating mechanism. Therefore a folic acid prescription for anemic thalassemia carriers with poor dietary intake can be beneficial.
Although SCD and thalassemia major do not need special treatment during the first 5-6 mo of life, several immigration countries have included HBP in the ongoing newborn screening (NBS) originally intended for treatment of metabolic diseases directly after birth[20-24]. Although NBS comes one child too late, one could expect some retrospective and prospective primary prevention for couples who had an affected or a carrier child. Unfortunately, after 7 years of NBS in the Netherlands no changes in the incidence of these severe diseases and in the requests for prenatal diagnoses have been registered[14].
To introduce a more efficient screening for primary prevention in multi-ethnic societies, one needs to identify healthy couples at risk before the birth of the first affected child. Moreover, one needs to choose the most logical moment, to provide the most consistent structure and to apply the most economically and socially convenient strategy[24]. If we look at the experience from decades of prevention in endemic countries[7-13], we see mandatory screening options before marriage which are not likely to become recommended in modern multi-ethnic societies unless prescribed by specific cultures. Screening when planning a pregnancy would be ideal but no structures are available to deal with different cultures at this stage except perhaps the scarcely experienced and reluctant GP’s. In fact, pre-pregnancy counseling is not available in most of the non-endemic immigration areas where little awareness is present in the daily healthcare structures and in the public. Putting up such a structure would take lot of efforts, lot of time and money. Regarding PGD, most countries offer this only in case of fertility problems and/or embryo selection when a suitable stem cells donor is needed for an affected child.
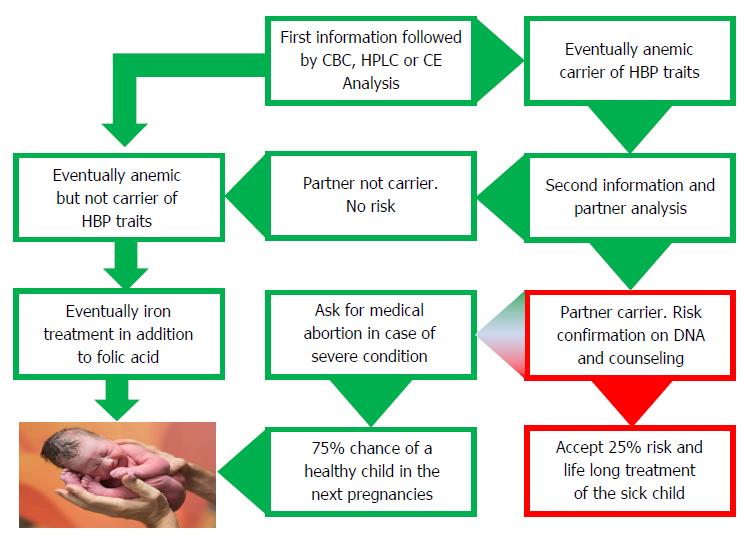
Screening early in pregnancy has been proven to be most effective and requested by well-informed couples in the endemic countries of Southern Europe and it is bound to be the most sensible options in most immigration countries (Figure 3). Screening early in pregnancy comes at a time when both parents are concerned with the good health of their progeny, when they are both generally involved and when the female partner has lesser chances to be stigmatized. Moreover, pregnancy care structures are usually well organized in most immigration countries where dedicated midwifes and gynecologists can provide information and offer screening and prevention[25]. The most practical option would then be to anticipate the first pregnancy visit and to offer hemoglobinopathy screening together with the rhesus and infectious diseases screening which is routinely offered to all pregnant women in most developed countries.
An important task for the obstetrician/gynecologist and for the laboratory analyzing the blood is to provide a thorough explanation. This can be done verbally and by using a standard letter when offering screening. Once the (pregnant) female carrier is diagnosed, short term partner analysis should be advised and to avoid anxiety it should be explained that being a carrier is not a disease but a recessive hereditary trait that only if present in both parents may affect their children with a chance of 1 in 4 causing a severe condition. Couples at risk should be made aware of the fact that the risk of 1 on 4 goes for every pregnancy and that having had one affected child does not mean that the next 3 should be healthy. Carriers should be reassured by explaining that each human is a carrier of several recessive traits and that knowing which one is an advantage that allows prevention if necessary.
Once the partner of a carrier of a relevant trait is found positive, then the putative couples at risk should be confirmed at the molecular level and counseled by a genetic counselor well informed about the severity of the diseases and the prognosis to be expected in the progeny. If the parents opt for prevention, prenatal diagnosis should be provided. Counseling should not be directive, neither toward treatment nor prevention, but based upon thorough and realistic information and should not be provided by doctors that may propose very expensive treatment without any hope of a definitive cure[3]. Parents should be counseled about the difference between treatment and cure. They should be made aware of the fact that the disease can be treated but cannot be cured and that, in spite of the best treatment, the severity of the disease will only progress until premature death. Bone marrow (stem cells) transplant (BMT) the only available “cure” today, may also fail causing severe graft versus host diseases and even when “successful” may leave the patient in a chronic post BMT condition requiring life-long treatment and monitoring.
Information on genetic risk can be interpreted in different ways in different cultures. In some cultures, when the pregnant female is diagnosed as a carrier and the male partner is asked to be checked, he might refuse to comply. In some culture the male might blame the female for getting a sick child. There have been cases of pregnant HbS carriers who had a child affected with SCD because the father did not show up, leaving the mother alone to take care of the sick child. It would therefore be ethically correct to offer prenatal diagnosis to female carriers also in absence of the father and in particular when the supposed father belongs to an ethnic group of high prevalence.
The author is grateful to Ing. Luca Giordano for providing the audio version of the “Core tip” paragraph.
P- Reviewer: Messinis IE, Mohammed Usta I S- Editor: Gong XM L- Editor: A E- Editor: Wang CH
| 1. | Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331-4336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 497] [Cited by in F6Publishing: 523] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 2. | Modell B, Darlison M, Birgens H, Cario H, Faustino P, Giordano PC, Gulbis B, Hopmeier P, Lena-Russo D, Romao L. Epidemiology of haemoglobin disorders in Europe: an overview. Scand J Clin Lab Invest. 2007;67:39-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 915] [Cited by in F6Publishing: 1066] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 3. | Koren A, Profeta L, Zalman L, Palmor H, Levin C, Zamir RB, Shalev S, Blondheim O. Prevention of β Thalassemia in Northern Israel - a Cost-Benefit Analysis. Mediterr J Hematol Infect Dis. 2014;6:e2014012. [PubMed] [Cited in This Article: ] |
| 4. | Giordano PC, Harteveld CL, Bakker E. Genetic epidemiology and preventive healthcare in multiethnic societies: the hemoglobinopathies. Int J Environ Res Public Health. 2014;11:6136-6146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Giordano PC. Prospective and retrospective primary prevention of hemoglobinopathies in multiethnic societies. Clin Biochem. 2009;42:1757-1766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Loukopoulos D. Haemoglobinopathies in Greece: prevention programme over the past 35 years. Indian J Med Res. 2011;134:572-576. [PubMed] [Cited in This Article: ] |
| 7. | Lena-Russo D, Erny N, Serradimigni F, Badens C, Aubinaud M, Merono F, Paolasso C, Mattei JF, Giraud F. [Genetic hemoglobin diseases. Prevention at centers for family planning and education of maternal-child protection in Marseille]. Presse Med. 1996;25:151-153. [PubMed] [Cited in This Article: ] |
| 8. | Angastiniotis M, Modell B, Englezos P, Boulyjenkov V. Prevention and control of haemoglobinopathies. Bull World Health Organ. 1995;73:375-386. [PubMed] [Cited in This Article: ] |
| 9. | Koren A, Zalman L, Palmor H, Zamir RB, Levin C, Openheim A, Daniel-Spiegel E, Shalev S, Filon D. Sickle cell anemia in northern Israel: screening and prevention. Isr Med Assoc J. 2009;11:229-234. [PubMed] [Cited in This Article: ] |
| 10. | Tarazi I, Al Najjar E, Lulu N, Sirdah M. Obligatory premarital tests for beta-thalassaemia in the Gaza Strip: evaluation and recommendations. Int J Lab Hematol. 2007;29:111-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Canatan D, Aydınok Y, Kılınç Y, Karakaş Z, Saşmaz I, Apak H, Sarper N. National thalassemia prevention campaign: the talotır project. Turk J Haematol. 2013;30:91-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | El-Beshlawy A, Youssry I. Prevention of hemoglobinopathies in Egypt. Hemoglobin. 2009;33 Suppl 1:S14-S20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Cousens NE, Gaff CL, Metcalfe SA, Delatycki MB. Carrier screening for beta-thalassaemia: a review of international practice. Eur J Hum Genet. 2010;18:1077-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Kaufmann JO, Krapels IP, Van Brussel BT, Zekveld-Vroon RC, Oosterwijk JC, van Erp F, van Echtelt J, Zwijnenburg PJ, Petrij F, Bakker E. After the introduction into the national newborn screening program: who is receiving genetic counseling for hemoglobinopathies in the Netherlands? Public Health Genomics. 2014;17:16-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Giordano PC. Strategies for basic laboratory diagnostics of the hemoglobinopathies in multi-ethnic societies: interpretation of results and pitfalls. Int J Lab Hematol. 2013;35:465-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Van Delft P, Lenters E, Bakker-Verweij M, de Korte M, Baylan U, Harteveld CL, Giordano PC. Evaluating five dedicated automatic devices for haemoglobinopathy diagnostics in multi-ethnic populations. Int J Lab Hematol. 2009;31:484-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Phylipsen M, Gallivan MV, Arkesteijn SG, Harteveld CL, Giordano PC. Occurrence of common and rare δ-globin gene defects in two multiethnic populations: thirteen new mutations and the significance of δ-globin gene defects in β-thalassemia diagnostics. Int J Lab Hematol. 2011;33:85-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Harteveld CL, Ponjee G, Bakker-Verweij M, Arkesteijn SG, Phylipsen M, Giordano PC. Hb Haaglanden: a new nonsickling β7Glu& gt; Val variant. Consequences for basic diagnostics, screening, and risk assessment when dealing with HbS-like variants. Int J Lab Hematol. 2012;34:551-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Mosca A, Paleari R, Ivaldi G, Galanello R, Giordano PC. The role of haemoglobin A(2) testing in the diagnosis of thalassaemias and related haemoglobinopathies. J Clin Pathol. 2009;62:13-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Bouva MJ, Mohrmann K, Brinkman HB, Kemper-Proper EA, Elvers B, Loeber JG, Verheul FE, Giordano PC. Implementing neonatal screening for haemoglobinopathies in the Netherlands. J Med Screen. 2010;17:58-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Streetly A, Latinovic R, Henthorn J. Positive screening and carrier results for the England-wide universal newborn sickle cell screening programme by ethnicity and area for 2005-07. J Clin Pathol. 2010;63:626-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Thuret I, Sarles J, Merono F, Suzineau E, Collomb J, Lena-Russo D, Levy N, Bardakdjian J, Badens C. Neonatal screening for sickle cell disease in France: evaluation of the selective process. J Clin Pathol. 2010;63:548-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Bain BJ. Neonatal/newborn haemoglobinopathy screening in Europe and Africa. J Clin Pathol. 2009;62:53-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Giordano PC, Rachmilewitz E. The Price of Mercy: Comment to the Paper Entitled “Prevention of Beta Thalassemia In Northern Israel - A Cost-Benefit Analysis” by Koren et Al. recently published in Mediterranean Journal of Hematology and Infectious Diseases. Mediterr J Hematol Infect Dis. 2014;6:e2014022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Kaufmann JO, Demirel-Güngör G, Selles A, Hudig C, Steen G, Ponjee G, Holleboom C, Freeman LM, Hendiks J, Wijermans P. Feasibility of nonselective testing for hemoglobinopathies in early pregnancy in The Netherlands. Prenat Diagn. 2011;31:1259-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |